In the midst of an influenza attack, B cells collaborate with various immune cells, subsequently branching into different defensive roles. One such role involves B cells evolving into cells that produce antibodies. Another significant role sees B cells transforming into lung-resident memory B cells, termed lung-BRMs, which are pivotal for respiratory immunity.

Contrary to the antibody-generating B cells that tackle the immediate infection, these enduring, stationary lung-BRMs journey from the lymph nodes that drain into the lungs. They establish a permanent presence, serving as the primary defense line, poised to swiftly generate antibodies during subsequent infections.
Grasping the process that gives rise to these lung-BRMs is crucial for enhancing flu vaccine formulations. The World Health Organization states that seasonal flu claims the lives of 290,000 to 650,000 individuals annually. However, flu vaccines demonstrate reduced efficacy in older adults, the demographic most vulnerable, in contrast to their younger counterparts. Moreover, there’s a pressing need for vaccines that can combat evolving virus strains more effectively.
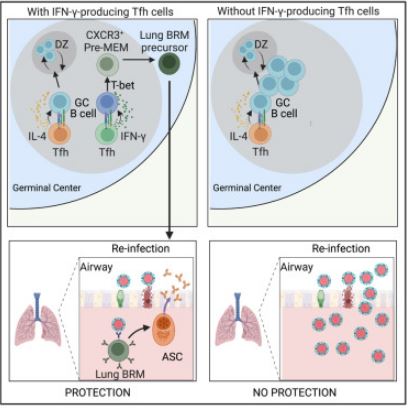
A recent study in the journal Immunity by André Ballesteros-Tato, Ph.D., and his team from the University of Alabama at Birmingham, utilizing a mouse model, revealed that the interferon-gamma emitted by T follicular helper cells (Tfh cells) post-influenza nasal infection is essential to set the B cell evolution towards lung-BRMs in motion.
During a flu outbreak, both Tfh and B cells are found in the germinal centers of the lymph nodes connected to the lungs. Memory B cells, which have undergone class-switching and are primed against the flu virus, start appearing in the lungs around the 10th day post-infection, peaking on the 30th day. The UAB researchers discovered that in the absence of Tfh cells or when these cells were neutralized by an antibody, the accumulation of lung-BRMs was hindered. This indicates the indispensable role of Tfh cells in facilitating class-switched-specific BRM responses to the flu.
The study further explored the function of Tfh cells, finding that the early differentiation of lung-BRMs post-infection was associated with variations in the Tfh cell response during the initial stages of the viral attack. The count of Tfh cells surged rapidly, reaching its zenith between days 10 and 15. By the 10th day, almost 40% of Tfh cells were emitting interferon-gamma, a figure that plummeted soon after.
Ballesteros-Tato and his colleagues deduced that the interferon-gamma production by Tfh cells was instrumental in the lung-BRM response during flu. Mice with Tfh cells incapable of producing interferon-gamma exhibited a stark reduction in the frequency and count of flu-specific BRMs in the lungs. Moreover, the lack of interferon-gamma-producing Tfh cells, resulting in fewer lung-BRMs, compromised the immune defense when these mice were subsequently exposed to a different flu strain.
The team also delved into whether the need for interferon-gamma signaling in lung-BRM development was intrinsic to B cells. Their findings revealed that mice with B cells devoid of the interferon receptor had a significant drop in the number of flu-specific BRMs post-flu virus infection. Furthermore, the transcription factor STAT1, known to be vital for optimal interferon-gamma signaling, was found to be essential. Mice lacking STAT1 in their B cells failed to accumulate flu-specific BRMs post-infection.
In terms of the underlying mechanisms, the researchers identified that the intrinsic interferon-gamma-STAT1 signaling in B cells within the germinal center of lung-draining lymph nodes elevated the expression of the T-bet transcription factor. This factor was essential for evolving into pre-memory B cells marked by the surface marker CXCR3. These cells then transformed into CXCR3+ memory B cells, which left the mediastinal lymph nodes and migrated to the lungs, becoming lung-BRMs.
Ballesteros-Tato commented, “Our findings underscore the pivotal role of interferon-gamma-producing Tfh cells in generating lung-BRM responses, offering fresh perspectives on the mechanisms that modulate germinal center B cell fate post-flu virus infection. This understanding is paramount for devising vaccine strategies aimed at eliciting robust lung-BRM responses, potentially offering enhanced protection against mutating virus strains.”
Sources
Arroyo-Díaz, N. M., Bachus, H., Papillion, A., Rosenberg, A. F., León, B., & Ballesteros-Tato, A. (2023). Interferon-γ production by Tfh cells is required for CXCR3+ pre-memory B cell differentiation and subsequent lung-resident memory B cell responses. Immunity, 56(10), P2358-2372.E5. https://doi.org/10.1016/j.immuni.2023.08.015